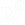Chronic inflammation is a harmful condition that might have a role in many diseases such as: asthma, rheumatoid arthritis, type 2 diabetes. Thus, anti-inflammatory properties of food supplements could be beneficial for human health, motivated by the fact that many anti-inflammatory drugs can cause testinal toxicity or other side effects. In order to examine potent anti-inflammatory potential, RAW 264.7 cells treated with nutrient supplement extract in the presence or absence of bacterial liposaccharide (LPS), which triggers the production and secretion of Nitric Oxide (NO) and inflammatory cytokines. RAW 264.7 cell line is a good system for such treatment since macrophages play an important role in immune regulation, initiation and resolution of inflammation. Major pro-inflammatory markers such as ΝΟ and tumor necrosis factor alpha (TNFa) or other secreted cytokines can be detected and evaluated in cell culture supernatants, either with specified assays (e.g Griess reaction) or with enzyme-linked immunosorbent assay (ELISA). More pro-inflammatory and anti-inflammatory macrophage phenotype markers can be validated in mRNA level, through Real Time PCR.
For nitric oxide measurement, RAW 264.7 cells are cultured overnight in 24-well plates and then are pre-treaded for 1 hour with different concentrations of extracts. Subsequently, macrophages are stimulated with 100 ng/mL LPS for 48 hours before nitric oxide measurement using Griess reaction. 50 μL of cell culture supernatant from each sample are transferred to a 96-well plate and mixed with equal volume of suldanilamide solution (1% sulfanilamide in 5% H3PO4) and mix is incubated at room temperature for 5 minutes, in dark conditions. Subsequently, 50 μL of NED solution (0.1% N-1-napthylendiamine dihydrochlorite in H2O) is added and absorbance is measured at 540 nm in an automated microplate reader. A sodium nitrite standard curve can be used for the evaluation of nitride concentration.
ELISA is a biochemistry immunoassay first described by Engvall and Perlmann in 1971 and is used to quantify antigen concentration within a sample. The basic principle is that flat-bottom 96-well plates coated with a capture antibody for the antigen of interest, bind the protein which is then detected with another antibody specified for a different epitope. Signal is produced after substrate solution addition. TNF-α cytokine is a good candidate for checking inflammatory actions of a food supplement using ELISA since it is secreted by macrophages in high levels after stimulation. The assay should be performed according to the manufacturer's instructions. Here we describe an example of a BioLegent ELISA MAX™ mouse TNF-α kit. Cell culture supernatants can be diluted or used as they are. It is generally proposed that when highly differential levels of target are detected, samples should be diluted accordingly. On the first day, plate bottoms are coated with Capture Antibody and incubated overnight between 2 ℃ and 8 ℃. The following day, plate is washed 4 times and Assay Diluent is added for blocking at room temperature for 1 hour with shaking. Plate is washed 4 times and Detection Antibody solution is added and incubated for 1 hour with shaking. Plate is washed 4 times and Avidin-HRP solution is added and incubated at room temperature for 30 minutes with shaking. Plate is washed well 5 times and TMB Substrate Solution is added and incubated in dark for 15 to 30 minutes or until desired color develops. Reaction is stopped with Stop Solution (2N H2SO4) and absorbance is red at 570nm. Control sample and standard curve should always be included in such assays in order to quantify applicably (link).
Another useful approach for evaluation of anti-inflammatory action of nutritional supplements, is the quantitative polymerase chain reaction for pro- and anti-inflammatory markers after LPS stimulation. RAW 264.7 cells are treated with LPS for 24 of 48 hours and then harvested and have their RNA extracted. Reverse transcription and Real Time PCR follows, using primers specific for M1 - M2 markers, depicting the pro- and anti-inflammatory phenotypic states of macrophages accordingly. The main pro-inflammatory markers are iNOS, TNF-a, IL-12, IL-1, IFNγ, mir155 while anti-inflammatory are Arginase 1, Ym1, Fizz1, IRAKm, mir146a.
The aforementioned cell culture assays can also be performed in peritoneal primary macrophages derived from mice fed with the nutritional supplements for a period of time. In particular, thioglycolate broth is injected to mice intraperitoneally, and macrophages are harvested 3 to four days later in Dulbecco's Modified Eagle's Medium (DMEM), centrifuged for 5 minutes in 1000 rounds per minute and resuspended in fresh DMEM under sterile conditions. Then, peritoneal macrophages can be plated, cultured and treated just as cell lines.