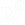Whether the process is fishmeal, enzymatic hydrolysis, or silage, the resulting three phases (water, oil, and solid) must be separated before further processing.
In the fishmeal process, a screw press performs the primary liquid-solid separation. The minced and cooked raw material is forced against a metal screen by a horizontally rotating screw conveyor. The liquid is pressed out through the screen and the solid phase (press cake) exits via the bottom of the machine at end of the screw (Figure 1.4.2.).

The press liquid is pumped into a (usually vertical) centrifuge (oil separator), separating the water (stickwater) and oil phases, also producing some sludge which may be blended back with the press cake. Most of the water in the protein-rich stickwater is removed on a falling-film evaporator before the concentrate is blended back into the presscake. The resulting fishmeal is then dried and milled. The oil phase, which is a valuable product, is further refined (purified) and may be used in aquaculture feed or human nutrition products (omega-3 oil or capsules).
Enzymatic hydrolysis, including the silage process, produces a greater amount of fine particulate solids. A screw press is unsuitable for this type of material, so a decanter centrifuge is typically used to separate the liquids from solids. A decanter centrifuge is a (usually horizontally) rotating cylinder with a conveyer screw inside that rotates at 5 to 60 rpm relative to the cylinder (which rotates at several thousand rpms, producing thousands of g-forces). The feed, containing liquids and particles, is pumped into the center of the centrifuge. The high g-forces cause the solids to settle to the inside surface of the cylinder. The solids are then moved by the screw, up a slope in the cone-shaped end of the cylinder, where the solids exit. The liquid builds up to a certain level and exits at the opposite end of the machine from the solids port. The water and oil are then separated in a vertical centrifuge (oil separator) as in the fishmeal process. Alternatively, 3-phase decanter centrifuges separate the solid, water, and oil phases in one process step (Figure 1.4.3).
Of the processes described previously, only enzymatic hydrolysis is normally used to produce concentrated protein products for human consumption. Instead of falling-film evaporators, crossflow nanofiltration (NF) is used to concentrate the protein-rich water phase. In addition to using less energy than evaporators, NF has the added benefits of removing monovalent salts and small molecules that contribute to off-flavors and odors.
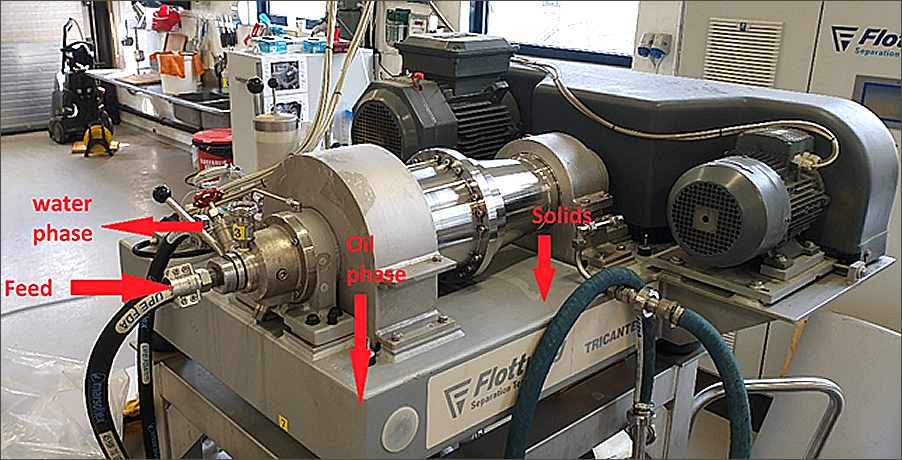
Crossflow filtration differs from so-called dead-end filtration (Figure 1.4.4). In crossflow filtration, the feed liquid flows perpendicularly to the filtration membrane and can pass through the membrane or exit the system though another route, producing two streams—permeate (the liquid that has passed though the membrane) and retentate (the liquid retained by the membrane). In dead-end filtration, which is the process most people envision when they read the word "filtration", the feed liquid is forced through the filtration membrane (by gravity or pressure) and the filtrate is the only liquid stream produced.
Crossflow filtration is often categorized into four types/levels: microfiltration (membrane pores in the micrometer range), ultrafiltration, nanofiltration (nanometer-scale pores), and reverse osmosis. Reverse osmosis membranes have pores so small that they only allow water molecules to pass while retaining sodium chloride and other larger molecules, producing permeate that is pure water. One step up in pore size from reverse osmosis, nanofiltration membranes allow passage of water, sodium chloride, and other (neutral or monovalent) small molecules. NF membranes are sometimes rated by the molecular weight cutoff (MWCO). MWCOs for nanofiltration membranes are usually between 200 and 1000 Dalton. If the water phase is to be concentrated by nanofiltration, most of the suspended particles must be removed beforehand to avoid fouling (clogging) the NF membrane. This may be done by crossflow microfiltration.
The NF permeate, which contains salt and other undesired compounds, is discarded (or the water from the permeate may be recycled by reverse osmosis) while the retentate (concentrate) is taken on to further up-concentration by an evaporator, or spray-dried directly. To optimize removal of salt and other small molecules, water may be periodically added to the feed tank (while recycling the retentate to the feed tank), causing more salt and other molecules to be removed along with the added water. This technique is called diafiltration.
The water phase from an enzymatic hydrolysis is typically around 5% dry matter (%DM) and may be concentrated to 25 %DM or more by nanofiltration. An additional drying step is needed to produce a protein powder, spray drying being the most usual.
Different technologies can be used for drying marine protein products, involving variable amounts of energy and temperature levels, steam or air, with vacuum or not. In our project inexpensive methods such as tray (air) drying will be used when appropriate, or more energy demanding ones depending on the quality specification of the products and the physical properties of the material. Freeze drying, spray drying, vacuum, steam and superheated steam drying are among the methods that are discussed in this module.
Selecting the right drying system is crucial for marine raw materials for many reasons, heat sensitivity is one of them. A poor choice of drying system and or drying conditions can have an adverse effect on physical and nutritional properties of the dried product and may give a reduction in biological digestible protein. The selection of a dryer for a particular raw material depends mainly on the type of end product, amount and type of moisture, drying kinetics, heat sensitivity, physical structure of the material to be dried, quality requirements of a dried food, and many other factors. Dryers are commonly classified based on the mode of heat transfer (e.g., conduction, convection, or radiation) and mode of operation (batch vs. continuous). For sheet forms or extruded food products, drying can be achieved on a belt conveyor or in a batch process by use of either conductive or radiative heat transfer along with convection and vacuum. However, pasty materials are conventionally dried using indirect rotary, paddle, or drum dryers and, recently, using beds of inert particles with some variant of the fluidized bed dryer as discussed previously for liquid suspensions.
In drying with superheated steam, the marine material is in direct contact with steam at temperatures above the condensation temperature. Superheated steam does not contain oxygen; hence, oxidative or combustion reactions are avoided. In addition, it eliminates the risk of fire and explosion hazard and the quality of the products tends to be better than that using a conventional hot air dryer. Superheated steam also allows pasteurization, sterilization, and deodorization of food products. This is particularly important for food and pharmaceutical products that require a high standard of hygienic processing. In addition, superheated steam drying provides higher drying rates in both constant and falling rate periods under certain conditions. Desirable organic compounds can also be contained using the superheated steam drying method. Mujumdar, 2007 has discussed the principles, advantages, and limitations as well as diverse applications of superheated steam drying technologies. Many foods and biomaterials are damaged at the saturation temperature of superheated steam. It may be necessary to include supplementary heat sources, for example, microwave radiation or conduction, to speed up drying rates at low steam pressures have provided a detailed discussion on superheated steam drying and dried various food materials using superheated steam.
Freeze-drying (also called lyophilization) comprises freezing a water solution or suspension of product then drying under vacuum. The water passes directly from the solid (ice) phase to gas phase before again freezing on the condenser coils or plates of the freeze drier. This process is called sublimation-going directly from solid to gas without passing through the liquid phase (more). The freeze-drying process has two phases: primary drying which removes frozen water through sublimation and secondary drying which removes non-frozen bound water. As the ice crystals disappear, they leave behind a porous mass that is (usually) easily crushed to a powder. Freeze-drying typically leaves a product with less than 3% moisture. Freeze drying is a gentle drying technique, as the product is not exposed to high temperatures. However, it is more expensive method than many other drying methods because of the energy required for vacuum and cooling, and the cost of the machine. Therefore, commercial applications of this technique in the food sector are limited to high-value products.
Spray drying is used when freeze drying of aqueous solutions is not economical or practical on a large scale. Perhaps the biggest advantage of spray drying is the quickness of the process. A solution or suspension of product can be dried to a powder in a matter of hours. It is also more amenable to large-scale production than freeze drying. A water solution or suspension is pumped into the spray dryer where it is sprayed into tiny droplets. At the same time, hot air is blown through the chamber causing the water in the droplets to evaporate. The resulting dry particles fall to the conical bottom of the spray dryer while moist warm air exits via another port. Spray-dried product also has little moisture, often less than 3%.