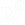In vivo testing of the bioactivity of nutritional compounds is fundamental before any safe conclusion can be drawn for the effectiveness (and safety) of a dietary supplement. Surely, in vitro or in silico trials often display important advantages, such as reduced time and inancial demands, compared to the corresponding in vivo experiments and can provide useful insights into the molecular mechanisms of action of the examined compound or, at least, give a rough estimation about its activity (i.e. anti-inflammatory). Extremely rarely are they sufficient, however. That is due to a plethora of reasons, a selection of which will be subsequently considered.
- Physiological process of digestion
To begin with, a determinant factor that is usually completely omitted during in vitro or in silico experiments is the physiological process of digestion. The bioactivity of an orally administrated nutritional supplement is immensely dependent on the bioavailability, distribution, metabolism, and excretion properties of its compounds. Frequently, it is noted that promising bioactivity of a substance in vitro is hindered in vivo because of its low bioavailability, as is the case with tea catechins or resveratrol. Many compounds are prone to extensive digestion by the enzymes of the gastrointestinal tract and do not even reach the absorption stage in the duodenum and jejunum or are subjected to chemical modifications affecting their activity. For instance, polyphenolic compounds which can act as inhibitors potent pancreatic lipase inhibitors exert their inhibitory effect in regards to their structural conformation as well as the degree of polymerization and elimination of glycosylation during digestion. Some compounds may also exert synergistic effects when combined with other dietary substances that could be lost at the level of a single substance, such as curcumin. Some others may exhibit desired effects by interacting with the digestion process itself, like polyphenols, which influence glucose metabolism by inhibiting carbohydrate digestion and absorption.
- Complex systemic responses to multifactorial pathologies
Especially when examining bioactivity in regards to such multifactorial pathologies like obesity and metabolic disease, intestinal inflammation, and cancer, where complex systemic responses are implicated and influence each other, including endocrine, immune and metabolic signaling, in vivo experiments are deemed mandatory. Taking as an example the immune system, it alone is thought to have a central role in all of the aforementioned diseases. For instance, obesity activates Kupffer cells, which then produce chemokines that trigger the accumulation of pro-inflammatory liver macrophages, which contribute to insulin resistance and hepatic steatosis. There is a variety of immune cell types with many different roles, assembling innate and adaptive immunity, whose interactions are vastly complicated. Even with co-cultures, in vitro conditions are yet nowhere near simulating real-life conditions of the perplexing immune system, therefore the effect of a nutritional substance on the above context should be also examined in vivo.
- Gender dependence
Gender itself seems to be pervasive in disease susceptibility, progression, and response to treatment. Gender differences can emerge from reversible or irreversible hormonal effects and from differential gene expression of the sex chromosomes, which, again, have a notable impact on major systems, such as the immune and metabolic system. For example, obesity-induced low-grade inflammation in the visceral adipose tissue (VAT), which contributes to the development of metabolic disease, is limited by estrogen in females, whereas in males is heightened. Therefore, a nutritional substance with an observed anti-inflammatory effect in vitro could potentially exhibit significant improvement in VAT inflammation in males but not in females in vivo.
- Intestinal microbiome
Intestinal flora, which is also neglected in vitro, is, nonetheless, a key factor by itself when testing dietary supplements. It is noteworthy that gut microbiota tend to differ between the two genders. The bioavailability and bioactivity of nutritional substances can be greatly impacted by the gut microbiome's metabolic activity during digestion. Dietary compounds shape gut flora's composition and influence the metabolites the microbiome excretes, through which the metabolism, the immune system, and inflammation, as well as the behavior itself of the host organism and even its circadian clock, among others, are modulated. For instance, gut bacteria metabolize choline and L-carnitine, which are provided mainly from animal sources, producing trimethylamine N-oxide, which, when elevated, the risk of developing pathologies like obesity or type 2 diabetes is shown to increase, too.
- Other factors
Finally, in vivo experiments collectively take into account so many other factors potentially affecting the effectiveness of a nutritional supplement in health or a disease context, such as strain, age, stress, physical exercise, feeding behavior, diet composition, the timing of supplementation, dietary-induced physiologic effects (i.e. satiety), including many more. Whatever that case, in vivo testing of the bioactivity of dietary supplementation is obligatory for the safe conclusion of the latter's actual effectiveness.
A vast number of animal models of metabolic disease have been developed, granted the high pressure of contemporary's pandemic of metabolic disease. An obvious categorization of such models would be the different animal groups used in obesity and diabetes research, that is, non-mammalian, rodent, large animal, and non-human primate models. There is a vast variety of genetic animal models, as well as non-genetic animal models, which result from interventions such as dietary, chemical or surgical. Needless to say, all of the aforementioned models present both advantages and drawbacks, and their choice strictly depends on the research question under investigation. Herein, rodent animal models and dietary treatments developed for the study of metabolic disease will be compactly presented, as their preclinical use on the field of metabolic disorders is the most extensive (more 2.4.2).
A classic approach to diet-induced obesity (DIO) and insulin resistance in rodent animals is the unconstrained feeding of the latter with high-calorie high-fat foods (referred to as high-fat diets, HFDs), possibly enriched in other dietary compounds, such as sugar or salt. This procedure simulates adequately the human obesity pathogenesis, involving progressive body weight increase and the consequential insulin resistance development.
Monogenic rodent models have arisen from mutations in the hypothalamic leptin-melanocortin feeding pathway, especially in genes encoding leptin and its receptors. The latter is the case with the historic mildly diabetic but critically obese ob/ob mice (C57BL/6J), which have a spontaneous mutation in Lep gene that prevents the secretion of bioactive leptin, and the relatively obese but severely diabetic db/db mice (C57BLKS/J), whose leptin receptor is mutated and defective. Both were initially developed in the 1960s and are used as preclinical models to this day. Various genes of the aforementioned pathway have been associated with human monogenic obesity syndromes.
Equivalent rat models also exist. Notably, the obese Zucker fa/fa rat has its leptin receptor trapped intracellularly due to a missense mutation in Leptr, resulting in weakened signaling. The Koletsky rat suffers from an inoperative leptin receptor stemming from a nonsense point mutation in Leptr. The ZDF rat presents malfunctioning β-cell transcription apparatus because of an autosomal recessive flaw.
Taking into account that GWAS studies have linked around 100 genes to obesity, polygenic models for researching such conditions are deemed necessary. An excessively used polygenic model of metabolic disease is the C57BL6/J mouse strain due to its susceptibility to hyperphagia-induced obesity when treated with HFD, although important heterogeneity in the increased body weight phenotype is noted, attributed mainly to epigenetic differences.
The New Zealand Obesity (NZO) mouse exhibits moderate hyperphagia, decreased physical activity, and energy expenditure, thus imitating human obesity. Plenty of other polygenic models are available, such as the TALLYHO/Jng mouse a non-insulin-dependent diabetes mellitus model, the DIO-sensitive or diet-resistant Sprague Dawley rat and the sand rat a nutrition-dependent early-onset obesity model. Genetic models for primary (instead of obesity-induced) pancreatic β-cell dysfunction, a key feature in the progression of diabetes, are also at one's disposal, like the Goto-Kakizaki (GK) rat or the FVFPBFDHom mice, which constitute a sexually dimorphic diabetic model13,14.
A classic experimental setup for the in vivo testing of nutritional supplements in a metabolic pathology context usually includes HFD feeding of a selected animal model for a minimum duration of 3 weeks. Key points that should be considered in order to minimize intra-group differences include the use of genetically identical animals of similar age, the same gender ratio among the different animal groups, a minimum of five animals per group and perfectly matched control groups, which should include both a HFD and a SD group. A typical dietary supplement assessment in regards to obesity, metabolic disease, and inflammation includes the following:
animal body weight is monitored tactically throughout the duration of the in vivo experiment, including pre- and after- fasting time points.
Food weight is measured before and after refill, in order to plot food consumption normalized to total weight.
For the GTT test, mice are fasted overnight (6-12h) and then injected i.p. with D-glucose (dextrose). Glucose measurements are received by mildly cutting the tail and measuring glucose in the blood with a glucometer at the following time points: 0, 15, 30, 60, and 120 min.
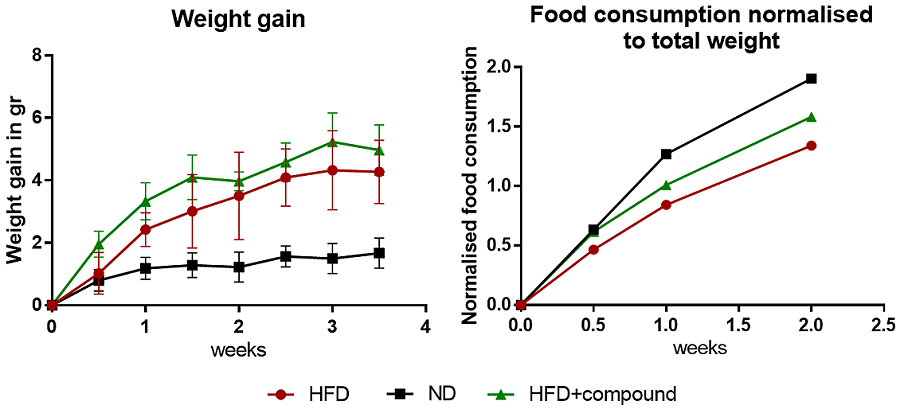
For the ITT test, mice are fasted overnight (6-12h) and then injected i.p. with insulin. Glucose measurements are received by mildly cutting the tail and measuring glucose in the blood with a glucometer at the following time points: 0, 15, 30, 60, and 120 min.
When mice are sacrificed, their abdominal fat tissue is isolated and its weight measured. The latter is normalized to total body weight. The same can be done for other tissues or organs of interest. Alternatively, the entire body composition can be measured using magnetic resonance imaging (MRI) and dual-energy X-ray absorptiometry scanning (DEXA).
Levels of inflammation are defined by quantifying markers of inflammation, mainly inflammatory cytokines, such as TNF-α or IL-6. Homogenized fat tissue is used for quantitative PCR or ELISA assay, and blood serum for ELISA assay.
A more detailed approach can be found here.